Tea Saponins
Category:
Cosmetic Ingredients
Tea saponin is a kind of natural nonionic surfactant with excellent performance, which has surface activities such as decontamination, foaming, emulsification, etc. Tea saponin is a secondary metabolite produced by camellia plants of Theaceae. It is kind of pentacyclic triterpenoid saponin and consists of sugar, ligand and organic acid ( chendel r, 1980 ). Up till now, seven saponin ligands of tea saponin have been isolated. These seven saponin ligands are all derivatives of oleanane, and the sugar part includes four kinds of glucuronic acid, xylose, arabinose and galactose. The organic acids are composed of angelica acid and acetic acid.
In the molecular structure of tea saponin sugar body is a hydrophilic group and sugar is connected with the hydrophobic group through ether bond. The hydrophobic group is composed of aglycon and organic acid connected in the form of ester bond. Thus it has the conditions to play a surface active role. The morphology of this hydrophilic-lipophilic structure at the two-phase interface can be expressed by a structural model, as shown in fig. 1. The molecular structure of tea saponin enables tea saponin to bind a large number of water molecules. as a result of surface adsorption, molecules on the surface of foam are arranged in a directional way, and many hydrogen bonds are formed between molecules with strong force. Thus tea saponin can make the liquid film in a high viscosity and stabilize the foam.Tea saponin is an excellent foaming agent.
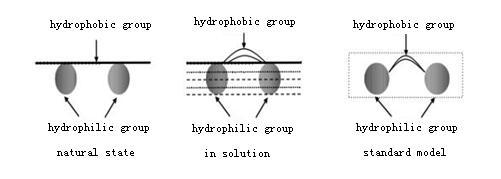
Fig. 1. Structural model of tea saponin molecules
- Characteristics of tea saponin as a surfactant
The surface activity of tea saponin can be described by HLB value, cloud point and CMC value.
HLB value is also called hydrophilic lipophilic balance value. Surfactant is amphipathic molecule with hydrophilic group and oleophilic group. The amount of size and degree of force balance between hydrophilic group and oleophilic group in surfactant molecule is defined as hydrophilic lipophilic balance value of surfactant.
Cloud point, a characteristic constant of nonionic surfactants, is influenced by the molecular structure of surfactants and coexisting substances. The water solution of surfactant will appear turbid with the increase of temperature. The surfactant solution will change from complete dissolution to partial dissolution. The temperature at which it changes is the cloud point. The cloud point ( CP ) is the temperature at which the phase separates will occur to homogeneous micellar solution of nonionic surfactant. Cloud point is a important physical parameter of surfactants.
Critical micelle concentration is the lowest concentration at which surfactant molecules associated in a solvent to form micelles.
HLB value measurement method: 1 g of soapnut extract is dissolved in 100 g water at 25℃ to get a translucent to transparent solution. Turbidity is measured by portable turbidimeter and HLB value range is measured according to turbidity.
Cloud point: 1 g of soapnut extract is dissolved in 100 g of water, heated in a water bath until turbidity occurs and the temperature at this time is the cloud point.
CMC ( critical micelle concentration ): prepare fresh solution of soapnut extract with purified water. Keep the temperature at 25℃. Measure the conductivity of the solution, plot the conductivity according to the concentration of the solution and measure the CMC value from the turning point of the curve.
Surface activity parameters of tea saponin
|
Surface activity parameters |
Result |
|
HLB value |
15~17 |
|
Cloud point |
>100℃ |
|
CMC value |
5.2g / L |
The data in this table are for reference only. the tea saponin will vary due to different sources of raw materials, different contents and different extraction methods.
Micelles:
Micelles in nature:
- The foam stabilizing effect of tea saponin
Tea saponin has good foaming ability, closely to SDS.
Compared with several synthetic surfactants participating in the study, the capacity to stabilize foam of is the best of tea saponin.And it has the longest half-life of foaming.
Tea saponin has good foam performance in high temperature, high electrolyte and wide pH range.
Foam of tea saponin has good oil resistance.
-
- Foaming ability of tea saponin
Preparation of 150 mg / kg hard water: 0.37 g of anhydrous magnesium sulfate ( MgSO4 ) and 0.50 g of anhydrous calcium chloride ( CaCl2) are weighed and fully dissolved in 5000 ml of distilled water.
Preheat the thermostat to 40±1℃ and keep the Roche foam thermostat at 40±1℃. Weigh 5.0g of the sample, stir to dissolve the sample evenly, fix the volume to 1L with 150 mg / kg hard water, and heat to 40±1℃. Use a 200 ml dosing funnel to draw some of the test solution and wash it along the wall of the foam meter. Then, put the test solution into the bottom of the foam meter and fix the volume to 50mL according to the standard scale. Then use a 200 ml quantitative funnel to suck the test solution, fix the the funnel at center position, put down the test solution.Immediately take down the foam height (H0) and then take down the foam height (H5) after 5 minutes. Repeat the measurement for three times to take the average value. H0 is used to measure the foaming ability of surfactants and. H5 is used to measure the stabilizing ability of surfactants.
Table 1. Roche bubble height at different concentrations of several foaming agents (cm) t :20℃
|
Concentration(g/L) |
SDS |
ABS |
AES |
Tea saponin |
||||
|
H0 |
H5 |
H0 |
H5 |
H0 |
H5 |
H0 |
H5 |
|
|
0.5 |
17.5 |
14.7 |
19.5 |
16.5 |
18.3 |
15.7 |
14.5 |
12.0 |
|
1.0 |
19.3 |
16.2 |
18.5 |
16.0 |
19.1 |
16.8 |
17.5 |
15.0 |
|
2.0 |
20.7 |
17.5 |
18.0 |
16.0 |
18.7 |
16.7 |
19.2 |
16.7 |
|
3.0 |
21.2 |
17.8 |
19.8 |
15.5 |
19.0 |
16.5 |
20.0 |
17.0 |
|
4.0 |
21.0 |
17.7 |
19.5 |
15.5 |
18.3 |
16.7 |
20.4 |
17.0 |
|
5.0 |
21.0 |
17.7 |
19.0 |
16.0 |
19.0 |
16.7 |
21.0 |
18.0 |
SDS:Sodium dodecyl sulfate;
ABS:Sodium dodecylbenzene sulfonate;
AES:Dodecyl alcohol polyoxyethylene ether sulfate
The foaming ability of tea saponin is close to that of SDS and its foam stability is better than that of SDS.
-
- Effect of pH on foam properties of tea saponin
Table 2. Foam height of tea saponin in solutions with different pH (cm)
|
pH |
1 |
3 |
5 |
7 |
9 |
11 |
13 |
|
H0 |
14.0 |
14.5 |
15.5 |
16.5 |
17.0 |
17.5 |
20.0 |
|
H3 |
9.0 |
12.0 |
14.0 |
14.5 |
14.7 |
15.5 |
17.5 |
|
H5 |
5.5 |
11.5 |
13.0 |
14.0 |
14.5 |
15.0 |
17.0 |
The foaming ability and foaming stability of tea saponin is increased with the increase of pH value of the solution. The foaming performance of tea saponin is poor in acidic solution ( pH < 4 ), but it can still foam. This may be for the reason that tea saponin exists in non-ionic form in medium with lower pH value. But when pH value is in a basic area tea saponin is mainly in anionic form in solution. Anionic surfactant can make the outer and inner surfaces of the liquid film of the foam take same electric charge to prevents the liquid film from thinning by water drainage due to the repulsive effect of the surface electric charge. That increase the foaming stability.
-
- Effect of temperature on foam properties of tea saponin
Table 3. Effect of temperature on foam properties of several foamint agents
|
Temperature ℃ |
Water quality |
Usage of foaming agent (g/L) |
Height of foam(cm) |
|||||
|
OP-10 |
CT5-2 |
Tea saponin |
||||||
|
H0 |
H5 |
H0 |
H5 |
H0 |
H5 |
|||
|
20 |
Tap water |
1.0 |
14.0 |
13.0 |
18.3 |
16.5 |
20.0 |
17.0 |
|
40 |
Tap water |
1.0 |
13.7 |
12.0 |
18.0 |
15.2 |
20.0 |
17.6 |
|
60 |
Tap water |
1.0 |
12.2 |
9.0 |
18.0 |
14.0 |
20.5 |
17.7 |
|
60 |
7%NaCl+1%CaCl2 |
2.0 |
1.5 |
1.0 |
16.5 |
4.5 |
18.0 |
12.0 |
|
80 |
7%NaCl+1%CaCl2 |
2.0 |
1.5 |
0.8 |
16.0 |
2.5 |
21.0 |
9.0 |
|
90 |
7%NaCl+1%CaCl2 |
1.0 |
1.3 |
0 |
11.5 |
1.5 |
24.0 |
7.0 |
OP-10:Alkylphenol polyoxyethylene ether;
CT5-2:Alkylphenol polyoxyethylene ether alkyl alcohol polyoxyethylene ether sulfate
The foaming ability of surfactant solution is poor at low temperature. With the increase of temperature the surface tension of surfactant solution decreases and foaming ability gradually increases. Most surfactants have the best foaming performance at 40 to 60℃. At higher temperature the viscosity of the foaming agent solution decreases, the foam tends to burst, and the performance deteriorates. However, the foaming ability of tea saponin increases slightly with the increase of temperature and the foam stability is also good, which may be related to the higher cloud point of tea saponin and the obvious thickening ability.
-
- Effect of electrolyte on foam properties of tea saponin
Table 4. Effect of electrolyte on foam performance t=80℃
|
Electrolyte concetration |
Usage of foaming agents (g/L) |
Height of foam(cm) |
|||||
|
OP-10 |
CT5-2 |
Tea saponin |
|||||
|
H0 |
H5 |
H0 |
H5 |
H0 |
H5 |
||
|
7%NaCl+1%CaCl2 |
1.0 |
1.2 |
0.5 |
14.5 |
4.5 |
21.0 |
9.5 |
|
10%NaCl+1%CaCl2 |
1.0 |
1.0 |
0.2 |
4.1 |
2.0 |
17.0 |
9.0 |
|
24%NaCl+1%CaCl2 |
1.0 |
0 |
0 |
1.5 |
1.0 |
15.0 |
13.0 |
OP-10:Alkylphenol polyoxyethylene ether;
CT5-2:Alkylphenol polyoxyethylene ether alkyl alcohol polyoxyethylene ether sulfate
The foam performance of OP-10 in electrolyte solution is very poor because the cloud point of OP-10 in 7%NaCl+1%CaCl2 solution is 42℃. The higher the salinity of water quality, the lower the cloud point of the foaming agent. The solubility of nonionic surfactant in the aqueous solution above its cloud point is greatly reduced and it is easy to form a new phase. at this time. And them not only the foam performance is very poor but also the defoaming effect.
CT5-2 is an anionic surfactant. The formed liquid film of foam has a electric double layer in film surface. The negative charges of electric double layers on the film surface repel each othe that increase the stability of the foam. However, when the concentration of electrolyte solution is increased, the diffusion electric double layer of the foam liquid membrane is compressed and the repulsion effect is reduced, the drainage speed in the membrane is accelerated, and the foam performance of CT5-2 is obviously decreased plus with the influence of temperature. Tea saponin has a similar situation. But because of its strong self-emulsifying ability and very high cloud point its solubility has not decreased significantly and it can maintain non-ionic and weak anionic surface activity. Therefore with the increase of water quality salinity the foam performance can be kept, even when salt is near saturation, it does not be precipitated, and the foam performance is still good.
-
- Oil resistance of tea saponin
Table 5. Foam performance measured by Waring Blender method
|
Foaming agents |
Temperature ℃ |
Usage of foaming agent (g/L) |
Kerosene % |
Water quality |
Foam volume (cm3) |
half-time (s) |
|
OP-10 |
25 |
10 |
12 |
10%NaCl+1%CaCl2 |
125 |
emulsion |
|
CT5-2 |
25 |
10 |
12 |
10%NaCl+1%CaCl2 |
125 |
emulsion |
|
Tea saponin |
25 |
10 |
12 |
10%NaCl+1%CaCl2 |
200 |
660 |
OP-10:Alkylphenol polyoxyethylene ether;
CT5-2:Alkylphenol polyoxyethylene ether alkyl alcohol polyoxyethylene ether sulfate
Grease or mineral oil can play the role of antifoaming agent. The resistance of surfactant to antifoaming agent can be tested by adding grease or mineral oil into surfactant solution. The foam produced by OP-10 and CT5-2 in the presence of kerosene will soon disappear and show an emulsified state. Under this condition tea saponin shows good foaming ability and foaming stability.
previous
Next
previous
Next








